Abstract
BACKGROUND
Asparaginase (ASNase) is an integral part of therapy for pediatric acute lymphoblastic leukemia (ALL). Pegylated E. coli ASNase (pegaspargase, PEG) and Erwinia ASNase (crisantaspase) are the two formulations currently in use in the USA. An ASNase activity of at least 0.1 IU/mL is the target necessary for adequate asparagine depletion from cells. Hypersensitivity reactions are the most common adverse events associated with ASNase. Two patterns of hypersensitivity are observed: acute, symptomatic allergic reaction or silent inactivation. In silent inactivation, there is in no clinically observed reaction, but immunologic clearance results in inadequate ASNase activity (< 0.1 IU/ml). Silent inactivation has been reported to occur in approximately 25% of patients receiving ASNase. However, these data are primarily from protocols where patients received native E. coli ASNase in induction before switching to PEG. Also, PEG was typically given via intramuscular injection in these studies. Our institution has exclusively used intravenous (i.v.) PEG for patients with ALL and lymphoblastic lymphoma (LLy) since 2012, and began monitoring ASNase activity in 2014. Our objective was to evaluate the incidence and pattern of hypersensitivity to PEG.
PATIENTS AND METHODS
We conducted a retrospective chart review of 221 patients with newly diagnosed ALL or LLy treated at our institution between 2014 and 2016. Of those, 96 were eligible for analysis based on the availability of at least 1 ASNase level, including B-ALL patients classified as high / very high-risk after induction, and all T-ALL / LLy patients (Table 1). PEG 2500 IU/m2 was given via 1-hour i.v. infusion per the schedule used in COG protocols. Routine premedication with antihistamines was not given. 125 patients were excluded due to low / average risk B-ALL, no ASNase levels, or having received alternate chemotherapy. ASNase activity was measured 1 week after PEG in the consolidation and delayed intensification phases of therapy. Silent inactivation was defined as an ASNase activity <0.1 IU/ml without a recorded allergic reaction. In addition to ASNase levels, we recorded the weight and body surface (BSA) for each patient before induction, and before and after the consolidation phase. We also recorded common adverse events associated with ASNase such as hyperglycemia (CTCAE > grade 3), liver dysfunction (CTCAE > grade 3), hypertriglyceridemia (CTCAE > grade 3), pancreatitis (CTCAE > grade 2), and thrombosis (CTCAE > grade 2). Descriptive statistics were created for patient and disease features, and Pearson correlation was used to evaluate association of ASNase levels to patient characteristics (SAS v9.4 Cary NC).
RESULTS
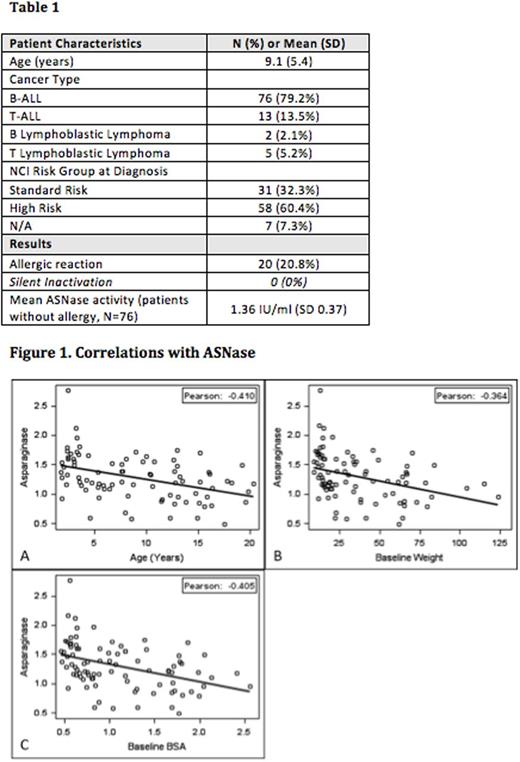
Patients included in the analysis had a mean age of 9.1 years; the majority had B-ALL (79.2%). Twenty patients (20.8%) had an overt allergic reaction after PEG. There were no patients (95% CI = 0 - 3.8%) with silent inactivation (Table 1). A total of 260 ASNase levels were recorded for an average of 2.7 ASNase levels per patient, with a maximum of 5 levels. The mean ASNase activity in patients without allergic reaction was 1.36 IU/ml (SD = 0.37). Age (rp = -0.41, p<0.001), weight (rp = -0.36, p < 0.001), and BSA (rp = -0.40, p < 0.001) all significantly correlated with lower ASNase activity (Figure 1). The most common ASNase associated adverse event was hepatotoxicity (N=70, 72.9%) with either an ALT >150 U/L and/or total bilirubin > 3 mg/dL, followed by hyperglycemia (N=22, 22.9%) with a glucose level >250 mg/dL, and thromboembolic event (N=11, 11.5%). Pancreatitis (N=4, 4.2%) and hypertriglyceridemia (N=5, 5.2%) were also observed.
CONCLUSIONS
Pediatric patients with ALL/LLy who receive i.v. PEG on the dose/schedule used in COG protocols do not experience silent inactivation. The mean ASNase activity in our experience is higher than reported previously. The incidence of overt allergic reaction is consistent with prior reports. Older patient age at diagnosis and higher baseline weight/BSA correlated with lower, but still therapeutic, ASNase activity. Hepatotoxicity was frequently noted in our series. These data suggest that routine monitoring of ASNase activity may not be warranted for pediatric patients receiving i.v. PEG as given on current COG protocols.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal